| Check out the SVPwiki | SVP Cosmology 2.3 |
A process which develops a "gas" from ordinary water invented by Yull Brown (originally a Bulgarian citizen who escaped to Turkay during communism time and later moved to Australia) and now deceased. The water is converted into a completely safe compressed stochimetric hydrogen and oxygen mixture. The flame of this gas under the right lighting conditions, normally almost transparently colorless, can be seen to possess a small blue cone, as it emits from a torch, with a longer, pale red-blue extension. Within its overall sheath are several distinct regions called "mantles". The most unusual property of the flame is that it is not formed as a set of explosions, as are ordinary flames, but as a set of implosions. Consequently, all classical theory about combustion products, highest temperature regions, and other specifics are up for revision. It is in the central blue cone of the flame, as opposed to its extension, that the novel combustion is sustained. This blue cone region separates the inner sustained vacuum from the continuously forming implosion products. The flame, upon application to an element or compound of elements, increases its temperature due to an interactive combustion property which is one of the unique characteristics of Browns Gas. There is no theoretical temperature limit to the flame as applied to materials as the local environment of the combustion will determine the extent of incremental calorific energy supplied and/or released. The temperature of the flame while in contact with only the surrounding air was measured to be 264 to 269 F (129 to 137 C). When the same flame was applied to the face of an ordinary building brick the temperature was measured at 3100 F. When the flame was applied to a tungsten wire the temperature was measured at nearly 6000 C.
 |
 Yull Brown |
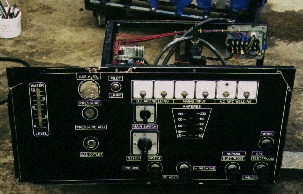 |
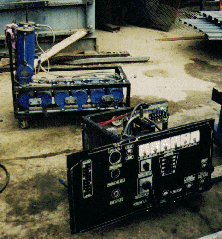
(These photos are of Yull Browns original gas generator now in our possession.)
The ratio 1,860:1 refers to the fact that when the gas is electrically sparked, it immediately returns to water. If the amount of gas sparked, and thus imploded could fill 1,860 units, then the amount of water produced by its implosion would then only fill one unit. The resulting space instantly becomes filled with a very high and particularly clean vacuum.
"There is no other method capable of producing such a gas. Browns Gas is a new product and there is no literature describing its properties which are sufficiently different from a combined molecular hydrogen and oxygen gas mixture, in 2:1 proportion, to be significant in industrial and commercial applications." Brown, 1979
Gas and its flame have been used in and exhibits characteristics:
1) Used in a car the gas combusts and emits water vapor as the only effluent in its exhaust.
2) A solid-state refrigeration unit in which temperature could instantly be changed with no freon or other refrigeration chemicals.
3) A room heater fueled with the gas will carbonize a strip of paper held near it but not create flames or smoke.
4) Used in an acetylene torch it singed hairs from a welders forearm but didnt burn the skin.
5) Flame from this gas can glaze concrete thus rendering it impervious to acids and other corrosives and greatly extending the concretes useful lifespan.
 6)
The gas when burned does not explode but implodes. "An intriguing
situation arises when a volume of Browns Gas is detonated because
the contraction in that volume which occurs is revolutionary in
character. Of an order of 1,860:1, the contraction can be defined as
an implosion, as opposed to an explosion." 1979
6)
The gas when burned does not explode but implodes. "An intriguing
situation arises when a volume of Browns Gas is detonated because
the contraction in that volume which occurs is revolutionary in
character. Of an order of 1,860:1, the contraction can be defined as
an implosion, as opposed to an explosion." 1979
7) When heating water in an iron basin using a torch if applied only to the water barely raises its temperature even after long exposure. The flame applied to the bottom of the basin raises the temperature of the metal so high, and so instantly, that the water boils away almost in the blink of an eye. When directed at a brick under the surface of the water, however, the flame can heat the brick as easily as though the brick was not water covered.
8) Steel, after treatment with the flame, is much more impervious to rust and before treatment.
9) The flame can fuse plastic to titantium.
10) Directing the flame at Cobalt-60 radiation was reduced by 70% in the sample.
11) Directing the flame at Americium the radiation was reduced 96%.
 |
 |
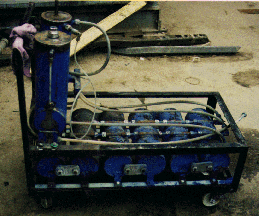
More photos of the Brown unit in
our possession.
 |
|
|
|
Browns Gas generators are
manufactured by Norinco, a Chinese
manufacturing concern, in four sized models and can be custom
manufactured in any size. These devices are also being manufactured by Eagle
Research.
Editors notes: There is evidence
Yul Brown did not invent this
system. It is claimed he borrowed the design and ideas from a Mr.
Rhodes of the
Henes Corporation. There are other articles on similar processes from
the 1940s. And lets not forget Keelys original
"ether liberation" work and devices from the 1870s. And his
most interesting work in dissociating
water into H and O using acoustic excitation.
Free Energy claims: We must add
the note it is unlikely Browns Gas will be used as a so-called free
energy source, in our estimation. The current manner BG is being
generated uses quite a
bit of electricity. Currently the cost of electricity exceeds the value
of the generated H. BG however can be exploded (as opposed to
its natural tendency to IMPLODE) by mixing air with it. I myself have
operated a VW with BG in this manner but only to show it could be
done.
Other Sources for Information on Browns Gas and Water as Fuel
See the SVP Forum discussion and collected information on Browns Gas and related subjects.
Keelys Water Dissociation:
http://www.svpvril.com/Water.html
James Allen Robey of Kentucky Water Fuel Museum (blog radio)
Dial In Number (718) 508-9430
March 31, 2007 - 7:00PM EST
http://www.blogtalkradio.com/waterfuel2007
Water as a Fuel - Browns
Gas
Andrew Michrowski
Planetary
Association for Clean Energy
100 Bronson Avenue # 1001,
Ottawa K1R 6G8 Canada
pacenet@canada.com
Abstract
The technology of producing a stoichiometric gas from an advanced alkaline electrolysis process as developed by Yull Brown has many clean and efficient applications, especially for heating, cooling, clean water production, water as an engine fuel and energy storage.
Keywords: Alkaline Electrolysis, Autonomous Housing, Browns Gas, Clean / Pure Water, Cooling, Combustion Efficiency, Desalination, Electrical Power Generation, Energy Storage, Healthy Homes, Heating, Heavy Oil Synthesis, Magnetohydrodynamic System, Materials Hardening, Oil Sand Synthesis, Toxic Waste Management, Vacuum Production, Water as a Fuel, Welding and Brazing.
1. INTRODUCTION
Our association is an international, inter-disciplinary collaborative network of advanced scientific thinking, founded in 1975 and based in Ottawa, Canada. Since 2004, we have begun acting in special consultative status with the United Nations Economical and Social Council. We would like to bring to the attention of countries aiming to turnaround the climate change situation without sacrificing either quality of life or socio-economic advances the potential technological choices offered by the systematic use of water as a fuel. Our network has followed and facilitated one such system since its inception: Browns Gas. Because so much research has been conducted with this technology, it is possible to describe many of its application with specifics. We believe that it is in the economical and political interest of nations to consider some of these applications in this decade.
Browns Gas is water separated into its 2 constituents by an advanced alkaline electrolysis process in a way that allows them to be mixed under pressure and then be burned together and safely in a 2:1 proportion. The process results in a gas containing ionic hydrogen and oxygen. When sparked, the gas recombines safely, by implosion, into water, collapsing in a vacuum/water ratio of 1,886.6/1.
2. APPLICATIONS
Three decades of research by the inventor, Yull Brown, an Australian citizen, have yielded numerous applications for the gas:
Applications of Browns Gas
(Further research may be required)
1. Air conditioning and cooling
2. Atmospheric motors
3. Cleansing of smokestack
4. Coal to Oil conversion
5. Curing
6. Deep-sea life support
7. Destruction of toxic wastes
8. Drying of fruit and legumes
9. Fuel Cell
10. Glazing and Kiln operation
11. Graphite production
12. Heating
13. Hydrogen production
14. Mineral separation
15. Nuclear waste decontamination
16. Ore separation
17. Oxygen production
18. Production of hard materials
19. Production of electricity
20. Pure water production
21. Silica conversion
22. Space life support
23. Underwater welding
24. Vacuum systems
25. Water pumps
26. Welding and brazing
______________________________________________________________________________
In this presentation, we focus on some applications that deal with renewable energy and optimization of environmental quality.
Browns Gas generators and some of the applications were first developed and manufactured in Australia. Production was transferred to the Peoples Republic of China at the inducement of its government, resulting in mass production of generators for national distribution. Important Chinese applications, besides welding and brazing, include water desalination, medical and toxic waste management and destruction, pharmaceutical production applications, and materials hardening. In 1996, the Chinese re-invited Yull Brown to build a Browns Gas system for deployment in automobiles. This particular technology transfer was interrupted in part due to ill health when he decided to return to his homeland, Australia, to spend the last months of his life.
Through the auspices of our Associations network, Yull Brown made arrangements for additional manufacturing facilities to produce generators and applications that would meet North American and European Union standards in Canada. One novel Canadian application is in synthesizing heavy crude and oil sands. Our Canadian colleagues are now successfully investigating applications in automobile engines, in optimizing the combustion of other fuels (wood, coal, natural gas, etc.) in to near complete burn and minimal emissions.
There is also the very convincing, but not yet test-proven on a large scale, case of using Browns Gas for the purpose of storing energy in such situations as excess hydro capacity, wind and solar energy by producing Browns Gas from electrolysis during slack demand periods and then using Browns Gas to produce electricity on demand during high-consumption periods. The efficiencies in both phases are very exciting.
The ready and limitless availability of water makes Browns Gas possibly the best carrier for solar energy and other alternative energy sources developed to this time. It has higher energy-conversion efficiency than hydrogen alone, which is conventionally considered to possess the highest conversion efficiency as fuel. Browns Gas is non-polluting -- it does not even emit the nitrogen oxides, which results from hydrogen burning. It is naturally recyclable -- the product of its burning is pure water. Browns Gas is adaptable, like hydrogen, to most of the existing energy utilization technologies, without any major modifications.
3. HEALTHY HOUSE APPLICATIONS
The illustration of the application of Browns Gas as a main source of energetics in a healthy and affordable house can help indicate the flexibilities offered by this fuel. A house would need a proportionately sized Browns Gas generator for all its basic requirements. These are as follows:
1) Heat. Attaching catalytic heaters to a supply of Browns Gas would provide heat for cooking elements and for space heating. The catalytic combustion (400 - 800∫C) resulting has the advantage of very significant heat loss reduction with, unlike all other available, no poisonous waste gases such as NOx. The range of temperature is determined by the control of the catalyst system itself. A ceramic carrier material with noble metals as catalyst operates at 400 to 600∫C and a power of 4 to 5 Watts per square centimetre. By using noble metal catalysts, no ignition is required for burning Browns Gas. On the other hand, in catalysts with a porous sintered metal, temperatures ranging from 700 to 800∫C are attained with power density of 15 to 20 Watts/cm2 but ignition is required. Such is the case of conventional gas burners. For cooking, hot plates using both types of catalytic techniques are commercially available. Contrary to hydrogen or hydrocarbon burning in catalytic cooking, which robs oxygen from the ambient medium, Browns Gas results in only pure water vapour with minimal humidity -- and no requirement for vents. Space heating by catalytic heaters using hydrogen and oxygen only, such as Browns Gas, are recognized to be more than 95% efficient.
2) Cooling. Water cooling and space cooling requirements can be provided by compressing Browns Gas and releasing it, on demand, by venting the result either directly onto the medium to be cooled or into the space to be cooled. A more efficient system might involve exposing a Browns Gas flame to a circulating freon (or like) gas tubing, not unlike the old method of applying lit gas lamps or paraffin wicks in the pioneer frigidaires.
3) Pure Water would be available on demand by re-conversion back to water.
4) Energy Storage System. One litre of water, with about 5 kW input generates 1,866 litres of Browns Gas that can be released to a chamber located up to 10 metres above floor height, which is linked to a flexible pipe connected to a water basin subject to ambient atmospheric pressure. When the chamber of Browns Gas is ignited with a spark, it creates vacuum by implosion. This triggers the atmospheric pressure to suction pump about 1,866 litres of water upwards the height of 10 metres to fill the container. The head of water can be used to drive an alternator for electrical energy if so required or desired. Other applications could include suction pumps, irrigation, etc. Under proper conditions, 1 litre of water at 10m has the potential to produce 98 Watts; 1,866 kg of water has the potential to generate 182.9 kW/litre of water consumed. The inherent inefficiencies of the various energy users will have to be considered for the final design configuration utilized.
Such a system has been operated for a period of 10 years. Browns Gas storage is over 98% efficient, as are current hydrogen and oxygen tank storage systems. Were the Browns Gas generation and storage system be replaced by a conventional bottled liquid gas supply, the total costs of operation are estimated to become 20% higher. So it makes more sense to have the gas generation system installed indoors.
Experience to date indicates that the Browns Gas generator, a commercially available alkaline electrolyser, unlike others, does not produce electrolyte creepage nor presents any difficulties in automatically controlled start-up procedures.
Browns Gas could fit in a house with a solar cell system. It would also replace the need for massive storage batteries, which present burdensome maintenance tasks for the average homeowner. A viable concept would involve a high-efficiency storage system integrating a Browns Gas generator and the distribution of gas to appliances (stove, refrigerator and air-conditioning units) and a DC/AC converter. Other arrangements are also possible.
The use of Browns Gas in such configuration for autonomous housing: 1) extends benefits in indoor air and water quality, 2) allows additional space heating and space cooling options, 3) potentially lowers sound levels, and, 4) reduces and/or eliminates some of the expensive elements or aspects of present systems. It would fit well with initiatives in remote areas to install housing in isolated communities that assure problem-free energy production, heating, air venting, clean water, grey water, sewage treatment. The combination of Browns Gas generation and energy storage system in such stand-alone block units should optimize this initiative.
4. HIGH-EFFICIENCY COMBUSTION
There is the question of the big picture. Existing combustion technology can be boosted from low efficiency to extremely high efficiencies by spraying Browns Gas onto flames, an application now being manufactured in China for waste and medical waste incinerators. Large-scale application of this fact can mean big advantages to those economies that are dependent on imported fuel supply. A similar context exists in the Federal Republic of Germany, where an econometric study by the University of Hagen explored the global implications of applying low-cost Browns Gas for heat and electricity generation (with both centralized and decentralized settings) and for the transportation sector. It recognizes that a phased implementation of such a system would be beneficial in terms of national budget because of decreased expenditures related to the environment, it would probably have lead to an increase in employment and greater use of the highway infrastructure by cars and could stimulate the economy with greater purchasing power. A similar and desirable consequence could be expected for many regions throughout the world.
The Browns Gas-generating alkaline electrolyser uses a mixture of sodium hydroxide with the supplied water to form an effective electrolyte with a measured conversion efficiency in the 90 to 95% range excluding cable and other system losses. The theoretical energy level of hydrogen/oxygen gas is in the range of 50,000 Btus per pound. Browns Gas has about 66,000 Btus per pound (and, with some proprietary technological priming, up to 210,000 Btus). If just 80% of this energy can be recaptured, it would be a significant improvement on the main problem with all variable power input systems, solar, wind, tidal, etc.: namely, energy storage. The gas-storage system development is of a very high priority in future developmental work in this area yet experience suggests that the off-the-shelf liquid petroleum gas technology storage system conveniently adapt themselves to Browns Gas storage. But, only large consumption requirements warrant further Browns Gas-specific developmental work, such a might be considered by large utilities.
Browns Gas could be used to increase the efficiency of fuel cells upwards from their current low levels, especially by providing an inexpensive source of hydrogen. This could prove to be very interesting for variable power input hydroelectric plants and wind-energy farms.
5. MAGNETOHYDRODYNAMIC ELECTRICAL PLANT
Also of interest may be the use of Browns Gas to energize the magnetohydrodynamic system -- an electrical plant of no moving parts. MHD converts hot gases directly into electricity. The MHD requirement is to have temperatures about five times higher than conventional power plants. Such temperatures are readily available with Browns Gas. A method using Browns Gas would require that the gas be burnt to produce plasma at the nozzle end of a conical shaped rocket engine surrounded by a strong magnet. The hot gas would be then seeded with an ionized alkali metal such as potassium or caesium to induce electrical conductivity, and thereby setting up a strong electric field. With the magnets, DC current would be generated very efficiently -- with an estimated improvement of about 20% over conventional systems.
6. PUSH-PULL RADIAL ENGINE
Medium-sized industries of many countries could develop an advanced push-pull radial engine that would optimize the peculiar physical properties of Browns Gas. It would be a kinematic arrangement of atmospheric pressure vs. Browns Gas-created vacuum will allow the creation of a push-pull engine to generate motive force; for example, driving pumps, ventilators, etc. Preliminary calculations show that such an engine will have very impressive characteristics and easily outperform the more exotic technologies such as Stirling engine generators and fuel cells. The proposed 3-cylinder radial engine has at least one piston doing work on the crank at any one time, which will maintain continuous rotation of the shaft. This type of engine has very good emission characteristics and a low vibration level during operation.
7. AUTOMOTIVE ENGINE FUEL EXPERIENCE
Yull Brown drove a number of cars on a variety of internal combustion engines, performing many measurements on them using his laboratorys fully instrumented dynamometer set-up. He has been officially monitored to drive 1,000 miles per gallon of water.
The staff of Electronics Australia magazine found that the usual internal combustion engine needs very little modification to run on Browns Gas. The main thing is the removal of the carburetor and its replacement by a pressure reducer and throttle valve. The only other change needed to the engine itself us re-timing to allow for the fact that the hydrogen-oxygen mixture has a higher flame speed that the normal gasoline-air mixture. There is also a positive improvement in engine life since the only product of combustion is water vapour, leaving no carbon build-up on plugs and valves and no corrosion on the exhaust manifold or muffler due to acid vapours in the gas. The engine runs cooler, due to the absorption of heat by the exhaust water vapour as it expands on exhausting from the cylinders. And there is no pollution. The exhaust feels like a warm steam.
Browns Gas cells produce about 340 litres of gas (13.6 cu. ft.) per kilowatt-hour (estimated at between 16 to 194 times cheaper than bottled oxy/hydrogen gases and between 7 to 58 times cheaper than oxy/acetylene gases, depending on the electricity rates and the bottling costs worldwide). Each kWh of gas produces about 5,650 kiloJoules on the basis of heat output. Compare this with the oil industry data of 34,983 kJ per litre. This means than 1 cent will provide between 300 and 706 kJ of Browns Gas whereas it would provide about 290 kJ of gasoline (at C$ .80/litre). A small gasoline car in Canada costs 3 cents/kilometre to operate, an electric car costs about 1cent/kilometre and a Browns Gas full-size car should run at 0.20 cents per kilometre.
At this point in time, for use in automobiles, the gas may have to be stored in useful quantities, in conventional gas bottles, even though the stored energy/weight ratio of about 4400 W/hr per kilogram is not as good as for gasoline (about 13,200 W/hr per kilo of gasoline). While the energy-to-weight ratio is about a third of gasoline, it is better than that for batteries in general. Lead-acid batteries range from 40 W/hr per kg up to 350 W/hr for the lithium-sulphur variety.
Yull Brown began experiments in metal absorption on metallic surfaces, particularly on least costly metals so that large amounts of gas could be stored easily and safely in quite small volumes. He envisaged that bottled Browns Gas - to run cars and trucks and even to generate home electricity - could be rented at about $75/week. Canadian research shows that it is feasible to use small battery-charged Browns Gas units, the size of large Coke bottles to run on board vehicles.
8. VACUUM-PACKAGING AGRICULTURAL APPLICATIONS
Since Browns Gas offers easy and low-cost vacuum production, it permits the picking of fresh foods in-the-field, in vacuum packs, enhancing preservation, and destroying infestation. The technology also provides a drying system that does not adversely remove the water content of many produce, naturally and quickly. This would help not only in saving the yields now lost to rot but also allow freer distribution of fresh agricultural produce over greater distances. A widespread application of this technique could decrease substantially of the use of expensive petrochemical spraying. Agricultural regions could sell more food, at less cost. The technology was successfully applied in Australia for orchards and tobacco growing.
9. CONCLUSORY REMARKS
Renewable energy in the form of stoichiometric hydrogen/oxygen Browns Gas should be considered, in our opinion, as one of the most promising future energy fuels for many nations. This is a reasonably mature and available technology. It also has its inherent and very important safety features. It is also inexpensive in terms of the primary fuel but also in terms of capital requirements and it can adapt itself to retrofitting large facilities such as our aging nuclear generation plants to continue to supply electricity in large quantities without adverse environmental impacts.
Our Associations collaborative network would be pleased to embark on a technology transfer program to steward interested parties into a viable energy strategy that most nations can afford.
References
Wiseman, George: "A Browns Gas Manual", Planetary
Association for
Clean Energy, Ottawa, 1997
Michrowski, Andrew (compiler): "The Browns Gas File: Water as a Fuel -
from the Associations Archives", Planetary
Association for Clean
Energy, Ottawa, 1998
From: Leslie Pastor <lrpastor@optonline.net>
Subject: [svpvril] Hydrogen: Novelty of Fact: Judging the FutureFor the moment, until other free-energy devices are allowed to become a reality, hydrogen is our best route to abundantly accessible and available fuel, it is all around us, covering this planet, drenching us with its splendid abundance. What is needed, is an energy policy that will provide everyone on planet Earth ubiquitous abundant sources of limitless forms of energy. Hydrogen appears to fit the bill. The only other abundant source of energy is the sun, followed by the energy from the vacuum.
China is the economic engine that is fueling our energy deprivations. She is rapidly accelerating her drive for dominance; via her drive to acquire via acquisitions all of the various types of fuels she believes is required to sustain her developing economy. She has received so much wealth from the United States, via her control of our debt-service, that, it is beginning to worry the American people. Thus it is imperative that the world seeks alternative abundant and ubiquitous sources of fuels now. We need to de-politicize the fuel markets. Making energy abundant once again, so that All can participate in its beneficence. This can only be accomplished, when fuel once again becomes abundant and inexpensive for everyone on this planet.
China is a totalitarian form of government. The United States is a republican form of Government, [we are not a democracy]. There is a huge difference in policy between these two nations. Americans, by and large, find it repugnant that the wealth of their nation and its people, is being used to prop up totalitarianism. While we admire the industry of the Chinese people, we are also aware, that it is literally destroying our own industrial base, right here in the United States of America. We resent that the centrist totalitarian state-capitalism of China is being used to further enslave its own people, rather than freeing them from their unnecessary servitude. We are well aware that none of the wealth accumulated by the government of China is reaching its own people, in the form of prosperity and an abundant lifestyle, and for that matter the same applies to the people of the United States. We recognize, that, China, is expanding her base and is using this "wealth" to control world markets and to capture via acquisitions and mergers what she cannot accomplish on her own. We also recognize that China is attempting to control the United States via her control of our debt-service. We realize that China will attempt to control United States foreign policy, by threatening to pull the rug, out from under our economy and currency, by threatening to financially destabilize our money-markets and thus causing the world to lose faith in the US Dollar, as well as collapsing our economy.
I place the blame for this on the members of the Russell Trust Association, at Yale University, who, have conspired to create our current dilemma. Prescott S. Bush Jr. and others have paved the way for this policy to take root. An investigation of the Russell Trust Association is long overdue. Their agenda squarely does not fit well with the American people. The Bush family [by and large] is responsible for our dilemma. It didnt have to be this way, at all, and the Bush family knows this to be true. For those who believe that this is hogwash, I invite you to read Antony C. Suttons book: Americas Secret Establishment, ISBN: 0-9720207-0-5.
Our monetary system is a negative system; it is a debt producing system. The US Dollar is a debt instrument. The Federal Reserve System is an engine of economic destruction. It doesnt have to be, nor remain this way, we may yet be able to reverse this policy, but it will require immediate selfless action on all of our parts. The powers that be have decided to equalize the standard of living of the entire planet, by lowering the standard of living of the wealthiest nations on Earth. This is a ruinous and deliberate policy of the control paradigm. The United States and other nations have got to free themselves from this policy, before it is much too late for all of us. We must work together, in fixing this ruinous policy, or we will not be able to work at all.
From my own experience, from several decades of research regarding our systemic problem, it has been my opinion that the control aspect regarding novelty of fact free energy systems is a two-fold dilemma. On the one hand you have a manifest marvelous Creation with infinite and complex design, having structure and balance, demonstrating order and symmetry, providing freedom and beauty for everyone in absolute abundance. While on the other hand you have a systemic creation of absolute control that fosters a deliberate policy of devastation and destruction for anyone who attempts to develop original free energy technological designs. Primarily, because truly free energy systems, would enable everyone on this planet to come, go, be, think, and do as individuals free from the control of governments, corporations, and each other.
The other side of the coin [control paradigm/novelty of fact free energy] pertains to novelty of fact discoveries that will truly enable all of the people of this planet to enrich themselves both intellectually and monetarily.
Browns Gas Research: http://www.nottaughtinschools.com/Yull-Brown/index.html
This is rare information on Yull Brown, alleged inventor of Browns Gas. This has been disputed: William A. Rhodes warlab@aztec.asu.edu claims to be the originator/inventor, not Mr. Brown. Stirling D. Allan has provided excellent research: http://www.freeenergynews.com/Directory/RhodesGas/ .
George Wiseman currently makes BG machines, he comments ref about Dennis Lees BG machine. George also sells the plans, and designs, so that you can build your own. George Wiseman provides significant data regarding this interesting subject-matter.
Jean-Louis Naudin has accomplished significant progress in verifying the MAHG [Mollers Atomic Hydrogen Generator].
This appears to be very significant research regarding hydrogen usage and might be the winner we have all been waiting for.
All the Best,
Leslie R. Pastor
PS: I have personally used several BG machines with two associates. What George Wiseman tells about them is accurate and compelling.
PSS: While you are on the http://www.freeenergynews.com/Directory/RhodesGas/ web page, you will notice UN Secretary General Kofi Annan [in a picture] inspecting the MAHG http://pesn.com/2005/06/26/9600116_Naudin_MAHG/ Jean-Louis Naudin has done excellent research http://jlnlabs.imars.com/mahg/tests/pultests.htm in this area, providing fascinating, accurate information. Viva la France.
Research: Hydrogen: BG; MAHG; Jean-Louis Naudin: Researcher Extrodinaire
Browns Gas
http://www.nottaughtinschools.com/Yull-Brown/index.html
http://www.eagle-research.com/index.html
http://www.watertorch.com/links/links1.html
http://www.watertorch.com/bghistory/hisbg1.html
http://www.masonstrains.com/MTbrownsGas.htm
http://www.amasci.com/weird/bgf1.html
http://www.rexresearch.com/hyfuel/ybrown/4014777.htm
http://www.padrak.com/ine/NEN_6_2_2.html
William A. Rhodes: [BG]
http://www.freeenergynews.com/Directory/RhodesGas/
http://www.keelynet.com/energy/oxyhyd1.htm
http://www.pureenergysystems.com/academy/papers/Common_Duct_Electrolytic_OxyHydrogen/
http://www.pureenergysystems.com/academy/papers/Common_Duct_Electrolytic_OxyHydrogen/index.html
George Wiseman
http://www.eagle-research.com/index.html
http://www.eagle-research.com/nopatent/patfree.html
http://www.eagle-research.com/browngas/whatisbg/whatis.html
http://www.eskimo.com/ghawk/h-o/books.htm
Jean-Louis Naudin [Paris, France]
http://perso.wanadoo.fr/quanthommesuite/synergierlvjln.htm
http://www.spacenews.be/dossiers/Lifters_120103/lifters_e.html
http://members.aol.com/jnaudin509/
MAHG: Free Energy From Atomic Hydrogen
http://jlnlabs.imars.com/mahg/index.htm
http://jlnlabs.imars.com/mahg/tests/pultests.htm
http://jlnlabs.online.fr/mahg/tests/mahg2a.htm
http://search.freefind.com/find.html?id=7894721&w=0&p=0
http://pesn.com/2005/06/26/9600116_Naudin_MAHG/
The Global Institute For New Energy Technologies
http://www.peswiki.com/index.php/Directory:GIFNET
http://gifnet.2advanced.com/
Flash From Zero Point: Jeane Manning
http://www.earthpulse.com/science/zeropoint.html
Research: An Aspect of the control paradigm
Prescott S. Bush Jr
http://www.nndb.com/people/007/000055839/
http://www.sourcewatch.org/index.php?title=Prescott_Sheldon_Bush%2C_Jr.
http://home.att.net/m.standridge/ChinesenSEC.htm
http://hackenbush.org/hackenblog/blogives/00000913.htm
http://www.rense.com/general28/skolpmp.htm
http://www.tylwythteg.com/enemies/Bush/bush15.html
http://www.sageworksinc.com/bod.asp
http://www.usatoday.com/news/washington/2002/02/19/usat-prescott-bush.htm
http://www.mbaec-cdc.org/desktopdefault.aspx?page_id=161
http://www.worldaffairsforum.org/about_us.htm
http://www.informationclearinghouse.info/article3352.htm
Prescott S. Bush Sr.
http://nhgazette.com/cgi-bin/NHGstore.cgi?user_action=detail&catalogno=NN_Bush_Nazi_Link
http://www.lib.uconn.edu/online/research/speclib/ASC/findaids/Bush_PS/collectiondesc.htm
http://fleshingoutskullandbones.com/P.Bush-Union_Banking/P.Bush-Union_Banking.html
http://www.enduro-disks.de/bushtory.htm
http://www.sourcewatch.org/index.php?title=Prescott_Sheldon_Bush
http://www.mbpolitics.com/bush2000/VestingExplain.htm
http://www.answers.com/topic/prescott-bush
http://www.tarpley.net/bush1.htm
http://www.tarpley.net/bush2.htm
Prescott Bush Resources, Ltd
http://www.cnn.com/interactive/specials/0009/democracy/bush.popups/family.tree/prescott2.html
http://alpha-stim.com/Information/Technology/Testimonials/Consumer/Testimonial/testimonial_118.html
http://www.hellogreenwich.com/YP/c_MANAGEMENTCONSULTING.Cfm
United States of America - China Chamber of Commerce
http://www.usccc.org/
http://www.usccc.org/newhome/newyear2005.htm
http://www.joeant.com/DIR/info/get/10930/22261
http://english.people.com.cn/200310/28/eng20031028_127032.shtml
American Chamber of Commerce - China
http://61.135.139.166/amcham/show/content.php?Id=278
Reference:
http://www.eskimo.com/ghawk/h-o/
http://www.newenergytimes.com/news/NET8.htm
http://www.svpvril.com/svpweb9.html
http://notes.nt.gov.au/lant/hansard/HANSARD6.NSF/0/676619d451ba261a692562e6001ca268?OpenDocument
http://www.amasci.com/weird/wsci.html
http://www.pureenergysystems.com/events/conferences/2004/teslatech_SLC/LarryOja/BrownsGas.htm
http://www.geni.org/globalenergy/policy/renewableenergy/index.shtml
http://www.epri.com/
http://www.newsmax.com/
http://www.worldnetdaily.com/
http://archives.cnn.com/2001/US/04/04/bush.uncle/
http://www.rationalrevolution.net/war/bush_family_and_the_s.htm
http://www.americandynasty.net/LATimes_article.htm
http://www.iff-ifoundfreedom.com/freedom/chinese.html
http://www.tbrnews.org/index.htm
http://www.geocities.com/CapitolHill/Parliament/2398/posts.html
The following article was located in one of the old magazines from our research library. It looks as though Mr. Brown was reading some of these old articles which may have given him some original thoughts on developing his Gas Generator. In any event the following material will help us understand what is happening in the ether generator. We are interested in Browns Gas because this gas is near equivalent to Keelys Etheric Vapor of the 1st order. The Browns Gas Generator we have been using has the below mentioned lye and iron particles in the solution and we have wondered about their role in the process. Now we know more....
by Raymond B. Wailes
Popular Science Magazine, June 1940
DOES your home laboratory face a temporary shutdown, because previous experiments have depleted the contents of your chemical bottles? If so, a visit to your kitchen, laundry room, and medicine chest will be worth while. You may find new materials in the form of such familiar and inexpensive household products as lye, washing soda, baking soda, and sugar.
The lye that grocery stores sell for a few cents a canful is essentially sodium hydroxide. While it contains about six percent of chemical impurities, these will not impair its usefulness for many home-laboratory purposes. Because it absorbs moisture from the air, and is gradually converted to sodium carbonate by atmospheric carbon dioxide, it must be kept tightly stoppered. You will find that it lends itself to an entertaining variety of experiments.
If anyone asked you how to make hydrogen gas, probably you would think first of using a metal and an acid. But did you know that lye, a powerful alkali, could be used in place of the acid? To try it, mix some lye with iron nails, tacks, or filings, in about equal proportions, and place the mixture in an upright test tube. Heat the tube gently at first, from all sides, with a Bunsen burner or a good alcohol lamp held in your hand. This will expel moisture from the lye.
At intervals, test the escaping vapors for hydrogen with a lighted match. If the match flame is extinguished, steam is still being driven off and the reaction has not yet begun. So continue heating, more vigorously. After a time the issuing vapor can be ignited momentarily, and will finally burn continuously. If you are using a small test tube, the flame will be at the mouth. In a larger test tube, of about 1" diameter, the flame will "strike back" and burn at the surface of the iron-lye mixture. It will be yellow, owing to the sodium compound, and not colorless, as the flame of sodium-free hydrogen would be. However, this extremely slight trace of impurity will make no difference if you use the hydrogen in tests.
The test tube employed in this experiment must be discarded afterward, as the hot lye will certainly attack the glass. But if you are attracted by this very inexpensive method of making hydrogen gas, you can use an all-iron heating vessel for the gas generator. To a piece of iron pipe 4" to 6" long, with an inside diameter of 3/4", attach a pipe cap of corresponding size. Fill the pipe with the lye-iron mixture. Then fit the other end of the pipe with a reducing coupling, and complete the assembly by inserting a length of 1/8" pipe, suitably bent, to serve as a delivery tube. When the chemicals are used up, the homemade retort is simply taken apart and refilled.
Here is another way to make hydrogen with lye. Dissolve the lye in water, and let the solution act upon scraps of aluminum in a flask. To start generating the hydrogen, apply gentle heat to the flask. The flame may then be removed, and the reaction will proceed briskly without further assistance. Zinc metal may be substituted for the aluminum in this experiment.
A recent article of this series ( P.S.M., May 40, p. 192) pointed out the handy trick of preparing any desired chemical compound by dissolving the carbonate in the corresponding acid. The hydroxide may likewise be used, and in many cases lye may be employed as a go-between to prepare it.
Suppose you have some copper sulphate, and want to make copper nitrate. Make a solution of the copper sulphate and add a solution of lye. This will form a precipitate of copper hydroxide. Wash it well by decantation, and then filter it off. Now dissolve the copper hydroxide from the filter paper in dilute nitric acid, using as little acid as possible. The result is a solution of copper nitrate.
If you desire copper acetate, instead, prepare the copper hydroxide as above and then dissolve it in acetic acid. The same procedure may be used for many compounds other than those of copper.
In certain cases, care must be taken not to use too much lye, or the precipitated hydroxide will redissolve. This applies to compounds of aluminum, zinc, chromium, lead, antimony, and tin. In preparing these hydroxides, add a small amount of lye solution; stir; let the precipitate settle; and then add a few more drops of the lye solution to the clear upper liquid. Repeat this procedure until a new addition of lye solution gives no precipitate.
You need not precipitate the hydroxides of copper, iron, cadmium, and manganese so gingerly, since they will not redissolve in an excess of lye solution. On the other hand, some hydroxides cannot be precipitated at all with lye solution. These include the hydroxides of calcium, barium, strontium, ammonium, and potassium.
Washing soda may be within handy reach, in your home; or, for as little as ten cents, you can buy quite a large box of it. Its chemical name is sodium carbonate. A fresh package will contain large crystals, in which each molecule of sodium carbonate has ten molecules of water attached to it. On exposure to the air, the crystals slowly crumble into white powder, losing three of the ten water molecules in the process. This phenomenon goes by the name of efflorescence. Though the powder seems perfectly dry to the touch, it still contains seven water molecules to each one of sodium carbonate.
You can easily show that sodium carbonate holds such a high percentage of water by heating it, and condensing the expelled moisture. Completely fill a test tube with washing soda - either the fresh crystals or the powder. Fit the tube with a one-hole cork, carrying an L-shaped length of glass tubing. With a short piece of rubber tubing, connect the end to a second L-shaped section of glass tubing, with one limb 8" to 10" long. Let this limb reach the bottom of an empty test tube immersed in cold water. This constitutes a miniature condensing system.
Gently heat the tubeful of washing soda with a soft or waving flame, and then increase the heat. You will plainly see steam coming from the crystals, and two teaspoonfuls or so of water will condense in the receiver.
The white substance left in the heated test tube is still sodium carbonate. However each of its molecules now has only one molecule of water attached to it, or no water at all, depending upon the temperature to which it has been heated.
Whatever its water content, washing soda (sodium carbonate) effervesces when treated with a dilute acid, releasing bubbles of carbon dioxide gas. Baking soda (sodium bicarbonate ) does the same, as you have observed if you ever tried mixing it with vinegar (dilute acetic acid).
Sugar makes another interesting chemical to experiment with. To produce a compound that probably is new to you, dissolve as much sugar as you can in some pure grain alcohol. Now add a strong solution of lye. A white precipitate of a chemical called sodium sucrate will be formed.
Place a razor blade in plain water, and it will become rusty in a few hours. Another blade, placed in a water solution of sugar, will show no sign of rusting.
"Oxygen bath salts," which liberate free oxygen in the bath water, have been placed on the market. Sodium perborate, which you may find in your bathroom cabinet, can be used as the chief ingredient of a similar homemade preparation. A suitable formula calls for 270 grams of sodium perborate, 6 grams of manganese sulphate, and 10 grams of cream of tartar. (This is roughly equivalent to 9 1/2 ounces of sodium perborate, one generous teaspoonful of manganese sulphate, and two teaspoonfuls of cream of tartar).
When two ounces or more of this mixture is placed in a bath tub filled with water, the sodium perborate decomposes and releases oxygen bubbles. The manganese sulphate acts as a catalyst, helping the reaction along without entering into it. Slight acidity, which favors the reaction, is supplied by the cream of tartar.
On a test-tube scale, you can demonstrate the same reaction in your laboratory, by adding several drops of a manganese sulphate solution to a sodium perborate solution. Oxygen gas is liberated when the two solutions mix.
Here is another old article about creating hydrogen and oxygen gas from ordinary materials. In this case there is a new twist. Please note the reference to rotating particles towards the end of the article. The described scenario is not unlike what we are building into the Dynasphere. Some of this material sounds a lot like Joseph Newmans work with his rotating magnons.
Popular Science - June, 1944
If this experimenter is right, his discovery will upset all our accepted ideas on this familiar force.
By Alden P. Armagnac
Can a magnet take water to pieces? No, say physics textbooks. Yes, says Prof. Felix Ehrenhaft, former director of the Physical Institute at the University of Vienna, who now carries on his research in New York. If he should turn out to be right his findings in the realm of magnetism promise practical applications as far-reaching as the dynamos, motors, transformers telephones, and radio that have stemmed from Faradays fundamental research in electricity.
For his "impossible" experiment, Dr. Ehrenhaft employs the simplest of apparatus. Two shiny rods of pure Swedish iron, sealed in holes through opposite sides of a U-shaped tube, resemble a setup familiar to high-school students for breaking up water into hydrogen and oxygen gases by passing electricity through it. And that is exactly what would happen if Dr. Ehrenhaft attached electric wires from a battery to the rods. But he does no such thing.
Instead, he uses the iron rods as pole pieces, or north" and "south" ends, of a magnet - either an electromagnet or a permanent magnet. Bubbles of gas rise through the twin columns of acidulated water, to be collected and analyzed. As might be expected, nearly all of the gas is hydrogen, liberated by a commonplace chemical interaction between the iron rods and the dilute sulfuric acid, one percent by volume, in the water. But the phenomenal part of the experiment is that oxygen also turns up, Dr. Ehrenhaft recently told the American Physical Society. To be specific, it is found in clearly measurable proportions ranging from two to 12 percent of the total volume of gases. When the gases obtained with a permanent magnet are separated, the larger proportion of oxygen is found above the north pole of the magnet. After rigorous precautions - including short-circuiting the magnet poles with wire, so that the poles will be at the same electric potential - Dr. Ehrenhaft concludes that there is only one place the oxygen can possibly come from. And that is from water decomposed with a magnet! Without a magnet, pure hydrogen is evolved.
There is an interesting sidelight to this experiment. A strong permanent magnet of the Alnico type suffers a marked loss of strength - say, 10 percent in 24 hours - after being used to decompose water, Dr. Ehrenhaft observes. In fact, makers of the magnets, which are supposed to last for years without material change, have viewed what happens to them with astonishment and dismay. But no fault lies with their products. Energy from an electric battery is used up in decomposing water, and it would be only reasonable to expect energy stored up in a permanent magnet to be drained likewise.
What gives the utmost significance to the reported feat of breaking up water with a magnet is the fresh evidence it offers for the existence of "magnetic current," or a flow of magnetically charged particles, which has been suspected by noted pioneers and which Dr. Ehrenhaft now maintains he has proved. Confirmation of this amazing discovery would point to a possible future rival of electric current, perhaps capable of being harnessed in undreamed-of ways.
Needless to say, the scientific world will require a whole lot of convincing, since Dr. Ehrenhafts conclusions flatly contradict long-established beliefs. As every schoolboy is taught, a magnet has a north pole and a south pole. Break it in two with a hammer, and each piece will have a north pole and south pole of its own. No law forbids you to imagine a magnet with only one pole, and the idea comes in handy in certain electrical and radio calculations. But as for actual fact, you cannot have one pole without the other, an experimenter named Peter Peregrinus believed, he demonstrated it to his satisfaction, using a loadstone, in the year 1269, and prevailing opinion has backed him up ever since. (As we know now, the loadstone that he floated on a platform in water simply turned until its north pole faced the south magnetic pole of the earth, and vice versa. It showed no observable excess of north or of south magnetism - and hence the conclusion that the two were always equal.)
But would the dictum of "no separate magnetic poles" still hold true in a far more delicate test - say, if you substituted microscopic particles of iron or other magnetic metals, as tiny as particles of smoke, for the massive chunk of rock that Peregrinus used? Dr. Ehrenhaft has tried it. In an air gap between the north and south poles of a magnet, he sets up what he calls a homogenous magnetic field, that is, with the lines of magnetic force absolutely parallel. In this field, he finds, the metal particles move toward the north or south pole, reversing their direction according to the direction of the magnetic field. On the particles, he concludes, there must be an excess of north or south magnetic charge. Expanding the terminology of Faraday, he calls the particles magnetic ions. They are the single magnetic poles shown at the lower right of the colored drawing. Instead of bearing plus or minus electric charges, as familiar ions do, they carry north or south magnetic charges.
Now, just as traveling electric ions form an electric current, why shouldnt traveling magnetic ions form a magnetic current? See for yourself another of Dr. Ehrenhafts startling experiments, and draw your own conclusions.
This time the heart of the apparatus will be a small glass cell, fitted as before with pole pieces of pure iron that dip into water containing one percent of sulphuric acid. An electromagnet, turned on or off at will energizes the poles. From a projector, a powerful beam of light converges upon the narrow gap between the pole pieces, and a low-power microscope, mounted horizontally, reveals what happens there. Adding a camera provides a permanent record.
You begin with the Magnet turned off. Looking into the eyepiece of the microscope, you see streams of bubbles rising from both pole pieces. They are of hydrogen gas, liberated by the same chemical action as in the first experiment.
Throw the switch that turns on the magnet, and the scene abruptly changes. Stopped dead in their tracks, some of the bubbles cling to the pole pieces. Others leave one pole and travel to the other. Dr. Ehrenhaft calls special attention to bubbles moving downward against their own buoyancy, impelled by some unseen force stronger than gravity.
Meanwhile a spectacular phenomenon has been developing - a miniature merry-go-round of gas bubbles between the faces of the poles and parallel to them. Incapable of being shown adequately in a time exposure, the effect nevertheless appears plainly as a white blur, when the upper magnetic pole is given a conical shape for photographic purposes. Visual observation, shows striking details. If copper particles, say, have been added to the acidulated water, they will rotate in the same plane as the hydrogen bubbles, but in the opposite direction. For both, the speed of the whirligig depends upon the strength of the magnetic field. Reverse the polarity of the magnet, and each set of particles spins in the opposite direction.
Here are no wild-eyed theories, but perfectly demonstrable facts. Any skeptical physicist has a standing invitation to see them with his own eyes at Dr. Ehrenhafts laboratory, placed at his disposal in the New York City quarters of the famous Carl Zeiss optical firm. How to account for the phenomena remains a challenge to science, unless Dr. Ehrenhafts conclusions are to be accepted. See how neatly they would draw an analogy between well-known electric effects and new-found magnetic effects:
Bubbles or particles that travel between pole pieces of a magnet behave just as if they were magnetic ions, or clusters of them - repelled by like magnetic poles, and attracted by oppositely magnetized poles. This corresponds exactly with the way that "electric" or ordinary ions interact with positive and negative electrodes. And as for the ring-around-a-rosy behavior of the hydrogen bubbles and copper particles, Dr. Ehrenhaft concludes that these are electrically charged particles - ordinary ions - rotating about a magnetic current. This would be an exact counterpart of the classical conception that magnetism rotates about a current-carrying electric conductor.
Now the staggering implications of Dr. Ehrenhafts observations begin to unfold. Existence of such a thing as magnetic current, once established, would pave the way for industries as gigantic as those that the discovery of electricity led to in its time. A "gold rush" for practical applications might be expected. Patents for them would command fabulous sums, since inventions employing magnetic current would be basic.
What form they may take, no man can foresee, and Dr. Ehrenhaft cautiously declines to hazard a guess. Yet a visitor to his laboratory cannot resist the temptation to let his imagination run free. New kinds of motors and generators? Better ways to transmit power? Transformers that will work on direct current instead of alternating current? Atom smashers? Radical methods of seeing things in the dark, and through microscopes and telescopes? Ways to tap power from the magnetism of the earth itself? And, in your home, substitution of magnetic current - who ever got a shock from it? - for electric current? Pure dreams, all of them, today - but some of them, perhaps, realities of 2044.
Before magnetic currents could be put in harness, of course, a myriad of questions about their behavior remain to be studied and answered. So far, no one knows whether they can be led through wires, like electric currents, as well as through conducting liquids. If so, the wires might be of entirely different materials than the best conductors for electricity. Likewise, the most effective insulators for magnetic current might be substances totally unlike those used for electrical insulators. The whole subject offers as vast a field for pioneering research as electricity did a century ago. And now, as then, an amateur experimenter puttering in his basement stands as good a chance of making an epochal discovery as does a distinguished scientists in a great laboratory.
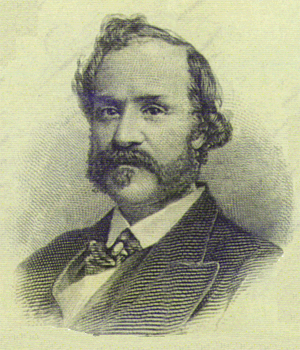 |
||
 |